Driving Breakthroughs: Target ALS Grants Empower the Next Wave of ALS Research
January 23, 2026
Looking back at 2025: A spotlight on Research We Fund
Target ALS is the largest and most impactful private funder of ALS research worldwide. Thanks to the generous trust placed in us by our donors, we can direct resources to the most promising ideas and emerging scientific leaders across the globe. Our funded projects have a high success rate, delivering meaningful results to advance ideas from early to late-stage research.
In 2025, we continued to expand funding opportunities, driving nearly $12M into innovation across 151 grants in three critical areas:
- Understanding ALS Biology: Advancing insights into the root causes of disease to identify new drug targets.
- Accelerating Drug Discovery: Supporting proof-of-concept studies that pave the way toward clinical trials.
- Developing Biomarkers: Enabling earlier diagnosis, identifying ALS subtypes, and monitoring disease progression with precision.
Every grant that Target ALS funds is tied to a deliberate strategy and a long-range vision for the field, ensuring that promising ideas move through the full research pathway: uncovering disease biology, identifying and refining new therapeutic targets or biomarkers, validating them in rigorous models, generating translational data, and preparing the strongest candidates for clinical development. Our funding allocation reflects where the strongest ideas emerge. The distribution of our funding isn’t set by quotas or percentages. It’s shaped by where the most compelling science is happening. Each year, we invest in the proposals with the clearest biological rationale, the strongest evidence, and the greatest potential to advance the field.
The Innovation Ecosystem Approach
By encouraging unpublished observations from grantees to be shared with the broader ecosystem at our Target ALS Annual Meeting, these funding opportunities create a pipeline of therapeutic targets and biomarkers that the entire ALS landscape is informed about. This ensures breakthroughs don’t stay trapped in individual labs but rapidly move toward therapies for people living with ALS.
We also bring a strategic, agile lens to deciding which funding calls to launch each year. Rather than repeating the same calls year after year, Target ALS scans the field, identifies the most critical bottlenecks, and tailors each funding call to deliver the greatest impact across the research ecosystem. We fund research through two mechanisms, collaborative consortia and individual investigator grants:
Collaborative Grants
Target ALS funds focused, multi-disciplinary consortia that cut through silos and speed the path from discovery toward clinical trials. These collaborations unite leading academic scientists with industry partners to focus on the same research problem together, including deciphering disease mechanisms, validating therapeutic targets, and discovering biomarkers. This mix matters. Academia brings novel ideas and foundational biology that push the field forward, while industry contributes by providing translational and drug development expertise, resources, infrastructure, and regulatory know-how that speed translation.
Individual Investigator Grants
Complementing our collaborative consortia, Target ALS invests in emerging scientific talent through individual investigator programs. These grants support early-career researchers with bold ideas, helping bring new ideas, cutting-edge expertise, technologies and fresh perspectives into the ALS field. This strategy strengthens the scientific pipeline, cultivates future leaders, and expands the community of innovators working toward effective treatments. Many of these scientists go on to become part of collaborative projects after being introduced to our network of global scientists, cementing their continued research in the ALS field. Programs include Springboard Fellowships, Early Stage ALS Clinicians, Neurology Residents, and New Academic Investigators.
A Robust Preclinical Pipeline
At Target ALS, we build and support a robust preclinical pipeline with purpose. Each project we fund is anchored to a clear scientific rationale and a strategic vision for advancing therapies through the full drug discovery and development pathway, from basic biology and target discovery to screening, lead optimization, and preclinical validation.
At every stage, the goal is to learn quickly and decisively. Sometimes that means recognizing which approaches should move forward. Just as often, it means identifying which ones should not. Both outcomes are successes, because each refines the path toward therapies that truly have the potential to benefit people living with ALS.
Our key focus areas and biological targets and pathways reflect this discipline: areas where the science is strongest and where new therapeutic opportunities are most likely to emerge and there is a meaningful path to clinical impact.
Featured Projects
Basic Biology Spotlight: Inside the Mystery of Sporadic ALS
A new Target ALS–funded consortium led by Clotilde Lagier-Tourenne is uncovering how innate immune pathways in neurons may drive sporadic ALS, the most common and least understood form of the disease. Partnering with Isaac Chiu, Brian Wainger, and Mark Albers, the team is revealing neurons as immune-active cells and identifying promising therapeutic targets already showing results in early studies.
Key take-away: By uniting experts across disciplines, Target ALS is advancing bold, collaborative science to unravel the mechanisms of sporadic ALS and bring new treatments closer to people with ALS.
Biomarker Development Spotlights: Tracking ALS at the Genetic Frontier
A Target ALS–funded collaboration between NeuroDex, Twilight Neuro, BrainEver Pharma, and NINDS is developing a blood-based biomarker to track transposable element (TE) activity: genetic elements that become overactive when TDP-43 loses function in ALS. By capturing neuron-derived extracellular vesicles and measuring TE levels, the team aims to monitor disease progression and treatment response across three therapeutic strategies: vaccine, protein replacement, and antiviral therapy.
Key Take-Away: This project could transform ALS care by turning a complex genetic signal into a simple blood test to guide and measure treatment effectiveness.

Cracking the Code of ALS: Cryptic Exons as Early Biomarkers
The Leonard Petrucelli (Mayo Clinic, US), Michael Ward (NINDS, US) and Pietro Fratta (UCL Queen Square Institute of Neurology, UK) consortium, in partnership with BioMarin, is advancing a powerful new class of biomarkers based on cryptic peptides: molecular signatures that emerge when TDP-43, a key RNA regulator, malfunctions in ALS. Their discovery of the HDGFL2 cryptic peptide in blood and CSF could enable earlier diagnosis and better tracking of disease progression, even before symptoms appear.
Key Take-Away:
By transforming TDP-43 dysfunction into a measurable biomarker, this collaboration is opening the door to earlier diagnosis, faster clinical trials, and a future where ALS is a treatable condition.
Drug Discovery Spotlights
Unlocking ALS: How Cell Biology and Genetics Are Shaping New Treatments
A global team including Johnathan Cooper- Knock (Sheffield, UK), Michael Snyder (Stanford, US), Ophir Shalem (UPENN, US) and Eran Hornstein (Weizmann, Israel), is uncovering how ALS begins at the cellular level and how to intervene. Using advanced tools like AI and high-resolution imaging, they’re mapping the early molecular changes that drive the disease.
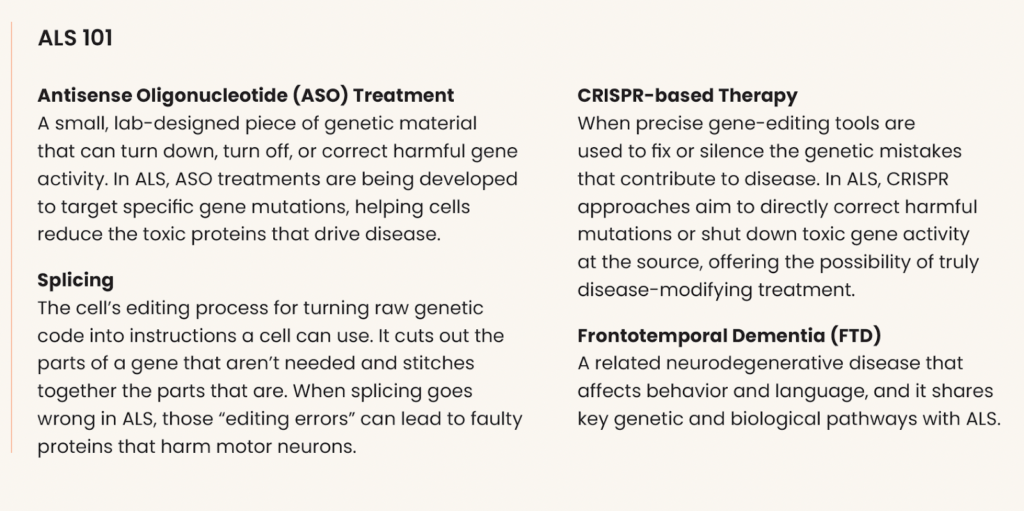
A key finding centers on CCDC146, a gene that becomes overactive in ALS and affects tiny sensing structures on cells. When researchers lowered this gene using an antisense oligonucleotide (ASO) treatment, motor neurons survived longer, highlighting a promising new therapeutic target.
The team is also studying how different structures inside cells behave in ALS, revealing common patterns of damage across genetic and non-genetic forms of the disease. These insights suggest that targeting what happens inside cells may be just as important as targeting genes themselves.
Key Take-Away: By illuminating how genes and cells break down, especially around the emerging target CCDC146, scientists are identifying new, more tailored pathways for ALS treatments.
Splice Correction of UNC13A: A New Frontier in ALS Treatment
A multi-institutional team including Ryan Morrie and Shila Mekhoubad (Trace Neuroscience), Aaron Gitler (Stanford), Sami Barmada (University of Michigan), and Noa Lipstein (FMP Berlin) is developing a promising and exciting new therapy that corrects RNA splicing errors in the UNC13A gene: a mistake seen in most ALS cases and linked to faster disease progression. By using ASOs, researchers have successfully restored normal UNC13A function in lab-grown neurons and genetically engineered mice. These corrections improved nerve cell communication, brain circuitry, and key survival markers. The team is now testing this approach in a mouse model that closely mimics human disease, laying the foundation for future clinical trials.
Key Take-Away: With early data showing restoration of neuron function and synaptic health, this work is a promising example of how understanding the genetic and mechanistic underpinnings of ALS can unlock transformative new treatments.
A New Modality in Motion: From Early Proof-of-Concept to Biotech Translation
At UCSF, Claire Clelland is pioneering a personalized CRISPR-based therapy for people with C9orf72- linked ALS and FTD. Her team has demonstrated that custom gene-editing tools can remove the toxic mutation from patient-derived neurons. Now, in partnership with Denali Therapeutics, they are developing a non-viral, IV-based delivery system to bring this treatment safely to the brain.
Target ALS first funded the early-stage, proof-of-concept work behind this ambitious CRISPR strategy, supporting Clelland’s team as they showed, for the first time, that a personalized gene-editing approach could remove the toxic C9orf72 mutation in patient-derived neurons. With those foundational results in place, we have now helped transition this effort into the biotech arena, where Denali Therapeutics is advancing the next phase of development using its innovative delivery platform.
Key Take-Away: With support from Target ALS, Clelland is advancing one of the first personalized CRISPR strategies for ALS, combining proven gene-editing success with Denali’s innovative delivery platform to move this bold science closer to people living with ALS.
Every day I think about the patients who are waiting on us. There’s not a second to waste.” – Claire Clelland, Ph.D., University of California, San Francisco
The Genetic Architecture of ALS
Understanding the genetic architecture of ALS is essential to unlocking its underlying mechanisms and developing therapies that can slow or stop the disease at its source. From studying large multigenerational families to expanding global genomic datasets, Target ALS–funded researchers are leading groundbreaking work to uncover the variants, modifiers, and molecular pathways that drive ALS and related diseases. Together, these projects reflect our commitment to diversity, data sharing, and discovery, ensuring that the genetics of ALS is understood in every population.
Unlocking Answers in ALS and FTD: Juliana Acosta-Uribe’s Groundbreaking Work in Colombia
In Colombia, where founder effects have revealed some of the world’s most important genetic insights into Alzheimer’s disease, Juliana Acosta-Uribe and collaborators are applying that legacy to ALS and Frontotemporal Dementia (FTD). Her team has identified several large Colombian families carrying the TARDBP Ile383Val mutation, which disrupts the TDP-43 protein, one of the key hallmarks of ALS pathology. Remarkably, some carriers develop ALS, others FTD, and others remain unaffected into old age. By combining genomic sequencing, organoid modeling, and longitudinal clinical studies, her work seeks to uncover the genetic and molecular modifiers that explain this variability.
Key takeaway: By studying these unique multigenerational families, Acosta-Uribe is uncovering how ancestral diversity shapes disease expression, revealing protective factors and therapeutic clues that could inform treatments for ALS and FTD worldwide.
ALS/FTD experts come together to study C9orf72 disease drivers: from genetic modifiers to toxic mechanisms
A new precompetitive consortium led by geneticist Rosa Rademakers (VIB), who first identified C9orf72 repeat expansions as the most common genetic cause of ALS and FTD, is uniting experts across both fields to unravel the drivers of C9 disease. Joined by Marka van Blitterswijk (Mayo Clinic), Adrian Isaac (University College London), and Renzo Mancuso (VIB), this team is working side by side to probe why symptoms vary so widely among people and families with the same mutation, from genetic modifiers to toxic mechanisms. This three-year initiative is designed to grow with science, with the potential to bring in industry partners or new collaborators to ensure that discoveries made in the lab translate into meaningful therapeutic development.
Key takeaway: By bringing ALS and FTD experts together around the leading genetic cause of both diseases, Target ALS is accelerating the path to targeted, mechanism-driven therapies.
Unlocking ALS Risk in South Asian Populations: Inside a Groundbreaking Genetic Study Led by India and the UK
Led by Henry Houlden (University College London), Atchayaram Nalini (NIMHANS, Bengaluru), and Vishnu Venugopalan (AIIMS, New Delhi), this first-of-its-kind Genome-Wide Association Study (GWAS) is mapping ALS genetics across India, where an estimated 100,000 people live with the disease but remain largely unrepresented in global studies. The study aims to enroll 2,000 people with ALS and 2,000 controls, integrating deep clinical phenotyping, longitudinal biofluid collection, and genomic sequencing to uncover both risk and protective variants.
Key takeaway: By bringing South Asian populations into the genetic landscape of ALS, this landmark study is closing critical gaps in representation, paving the way for precision medicine, equitable trial design, and globally relevant therapeutics.

Related News
Eric Dane | 1972-2026
February 20, 2026

Inside a Global Effort to Understand ALS Resilience
January 27, 2026
