
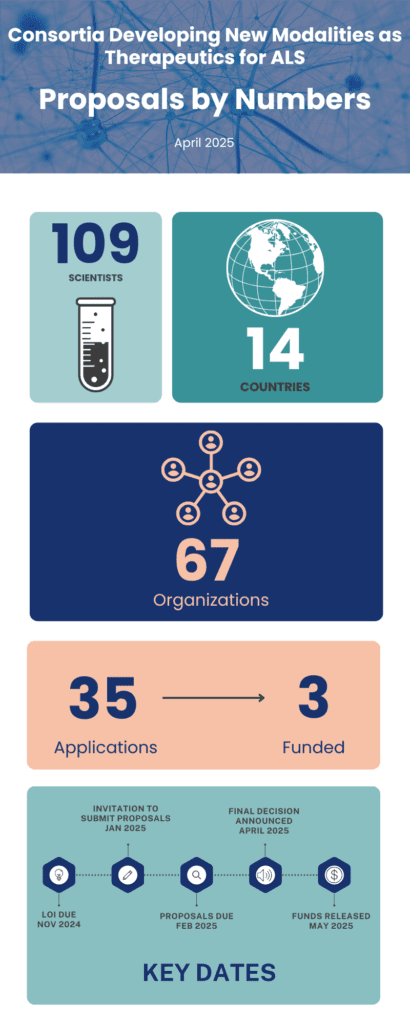
Three research consortia have been awarded Target ALS funding this April to develop next-generation gene therapies for ALS through our latest grant initiative, Consortia Developing New Modalities as Therapeutics for ALS.
This competitive initiative was designed to accelerate cutting-edge research aimed at developing novel DNA- and RNA-targeting therapies for both sporadic and familial ALS. The selected teams represent powerful collaborations, including between academia and industry, uniting complementary expertise to advance technologies with the potential to transform ALS treatment.
What This Grant Supports:
Each funded consortium will:
- Optimize drug-like properties of large molecules targeting RNA or DNA.
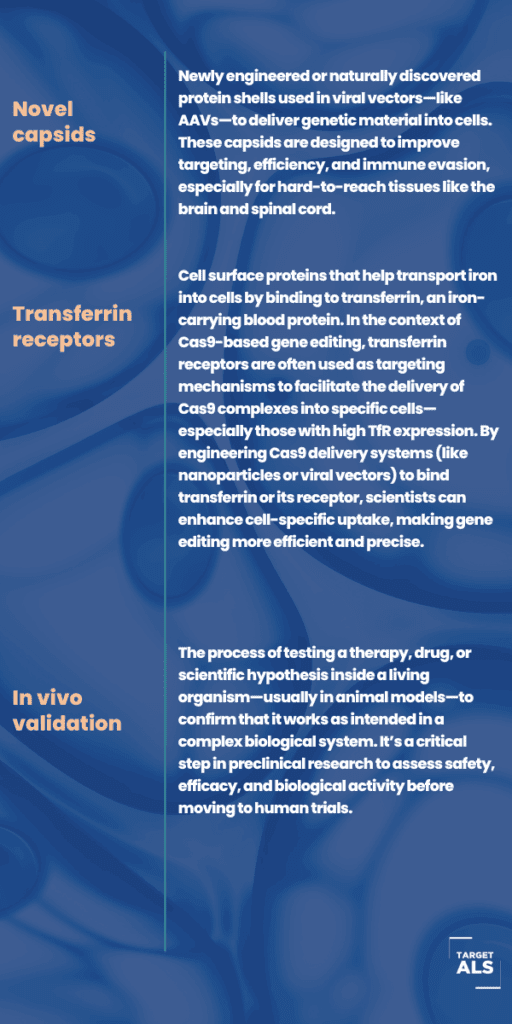
- Investigate next-gen therapeutic platforms, such as antisense oligonucleotides, siRNA/disiRNA, CRISPR-Cas9, and CRISPR-Cas13.
- Develop enhanced brain delivery strategies using transferrin receptor-mediated blood-brain barrier penetration, and other innovative systems.
- Focus on genetic targets associated with ALS, with the goal of moving promising molecules from the bench to in vivo validation.
Why This Matters:
It is well established that targeting the genetic cause of a disease can significantly improve patient outcomes, as shown by treatments like Tofersen and Nusinersen. However, to be truly impactful, these therapies need better “drug-like” properties—ideally, they should be taken conveniently at home as a pill or administered infrequently in a clinical setting via intravenous (i.v.) or intrathecal (i.t.) injection. Once delivered, the drug must reach all affected cells in the brain and spinal cord, effectively targeting the disease at its source. It must also be selective for the intended gene and demonstrate a strong safety profile. Many of the newer technologies being developed still face major challenges in meeting these criteria. With this grant, Target ALS aims to fuel the discovery and development of the next generation of gene-based therapies—ones with the power to meaningfully alter the course of ALS.
Routes of drug administration:
i.v. (intravenous): This means the drug is delivered directly into a vein, typically using a needle or catheter. It’s one of the fastest ways to deliver medications or fluids into the bloodstream, ensuring rapid effect.
i.t. (intrathecal): This refers to administration into the spinal canal, specifically into the cerebrospinal fluid (CSF) that surrounds the brain and spinal cord. It’s a much more targeted and invasive method, often used for medications that need to reach the central nervous system, such as certain chemotherapy agents, antibiotics, or in some cases, therapies for neurodegenerative diseases like ALS.
These projects exemplify our commitment to fostering cross-sector collaboration. Each consortium is composed of two to four synergistic labs, including partnerships with biotechs and pharma partners.
We’re honored to support their work and look forward to the breakthroughs that lie ahead. Grantees will also join the broader Target ALS community at our Annual Meeting in Boston—a key moment to share progress and spark new ideas.
The future of ALS therapy is being built—together.
2025 Novel Modalities Consortia Grantees:

Project Title: Nonviral transferrin-mediated Cas9 delivery to the CNS enabling CRISPR gene therapy for C9orf72-ALS
Researchers: Claire Clelland (UCSF, leader), Neel Singhal (UCSF) and Chris Koth (Denali Tx)
Project Summary: This project aims to combat the challenge of delivering CRISPR-Cas9 gene editing therapeutics to the central nervous system for ALS and other neurodegenerative diseases. Researchers at UCSF have developed a CRISPR-based approach that could treat the most common genetic form of ALS linked to the C9orf72 gene. However, one of the biggest outstanding challenges is delivering this therapy safely and effectively to the brain and spinal cord. To overcome this, the project brings together experts from UCSF and Denali Therapeutics to develop and optimize new, nonviral delivery systems. These advances could help make powerful gene therapies accessible for people living with ALS and other neurodegenerative conditions, allowing patients to take drugs through an i.v as opposed to surgery.

Project Title: Advancing RNA-targeting CRISPR modalities for treatment of FUS-linked ALS
Researchers: Thomas Gaj (U Illinois, leader) and Neil Shneider (Columbia U)
Project Summary: More than 50 different mutations in the FUS gene have been linked to ALS. These mutations are thought to make proteins that are harmful to cells, so finding ways to reduce or fix the faulty proteins could offer a promising path to treatment. This project brings together a team of experts to develop CRISPR-based tools to remove or correct mutated FUS. The goal is to cleanly replace only the mutant portions of the transcript, while avoiding the removal and elimination of the healthy transcript, creating targeted therapies for people with FUS-related ALS, with potential benefits for other types of the disease as well.
Project Title: Developing a Dual Targeting siRNASO Oligonucleotide Therapy for Amyotrophic Lateral Sclerosis Patients
Researchers: Evangelos Kiskinis (Northwestern U, leader), Jonathan Watts (UMass Chan)
Damon Wang, Joseph Klim (NuCyRNA)
Project Summary: Gene silencing with oligonucleotide-based therapeutics like ASOs is a promising treatment strategy for diseases like ALS, but there are still major hurdles to making it more effective. One challenge is that current therapies often have a low safety margin, and another is that targeting just one gene affected by TDP43 pathology may not be enough to make a real difference. This project tackles both issues by using a novel oligonucleotide technology that targets RNA in both the nucleus and cytoplasm. This dual-action approach allows for safer and more efficient treatment. This project specifically will develop novel oligonucleotide therapeutics that address both loss- and gain-of-function problems caused by TDP-43 pathology.
About our Review Process
At Target ALS, we hold fairness and transparency paramount in our review process. The Target ALS Independent Review Committee (IRC) makes all research funding decisions without involvement from the organization’s staff or leadership, ensuring every application receives a fair evaluation. The IRC is comprised of experts across scientific disciplines from both industry and academia, reflecting the evolving nature of ALS research. To avoid any possible conflicts of interest, no member of the IRC can apply for or receive Target ALS funding for their own work. Members on the IRC abide by a comprehensive conflict of interest policy and are all under confidentiality agreements.
For more detailed information about the grants and the application process, please visit our Grants page.
Our Vision and Commitment
At Target ALS, our vision is to realize a world where everyone lives. We are driven by a sense of urgency, knowing that every day counts for people diagnosed with ALS. Currently, there are no treatments on the market that promise to make sporadic ALS a manageable disease. We are committed to funding cutting-edge research and fostering collaborations that will bring us closer to effective treatments for all forms of ALS.Together, we continue to strive for a future where ALS is no longer a life-threatening diagnosis. Thank you to all the applicants for their dedication and hard work. For more detailed information about our grants and the application process, please visit our Grants page.





